100 years after the advent of TB vaccines, formulations vary widely

Each year, more than 100 million newborns around the world receive vaccinations against Mycobacterium tuberculosis, or TB, which infects about one-quarter of the world’s population. Facilities across the world produce several different formulations of these vaccines, known as Bacille Calmette-Guérin (BCG) vaccines. These are given interchangeably, yet new research from the Precision Vaccines Program at Boston Children’s Hospital calls that practice into question.
The study, which was published in the journal Vaccine, shows that BCG vaccines vary widely in their characteristics, including their ability to activate cytokines, potent elements of the immune system response.
“We found that licensed BCG vaccines differ dramatically, raising fundamental questions about whether the quality of these vaccines are equivalent and should be considered interchangeable,” explains co-senior investigator Ofer Levy, MD, PhD, director of the Precision Vaccines Program.
A global health threat
According to the World Health Organization (WHO), an estimated 10 million people, including nearly 1.1 million children, became ill from TB in 2018, and nearly 1.5 million people died from it.
TB infection in babies is particularly serious, often causing sepsis, meningitis, and, frequently, death. For that reason, many newborns receive TB vaccination in areas where the disease is common.
Vaccines against TB were first introduced in 1921. Today, more than 14 different BCG vaccine formulations are used, with five WHO-approved products dominating globally. All BCG vaccines use live, but altered or attenuated, Mycobacterium bovis (a bacterium related to TB) to spark the immune system to protect against TB.
BCG vaccines strengthen overall immune response
Previous research has shown that BCG vaccination not only protects against TB, but boosts the overall immune system, in what is called an ‘off-target’ effect.
“BCG vaccine is a very interesting vaccine because it has been found to boost protection against other infections, bacterial and viral, that are very common in newborns and young infants,” says Asimenia Angelidou, MD, PhD, a neonatologist at Boston Children’s and the study’s first author. “And it may be doing that by revving up the innate immune system.”
One recent study from the Precision Vaccines Program found that injecting BCG along with the hepatitis B vaccine strengthened the immune response to hepatitis B. “But until now, we have not directly compared these BCG vaccine formulations side-by-side for any standard measures of immunity or protection against TB,” says Levy.
Comparing BCG vaccines
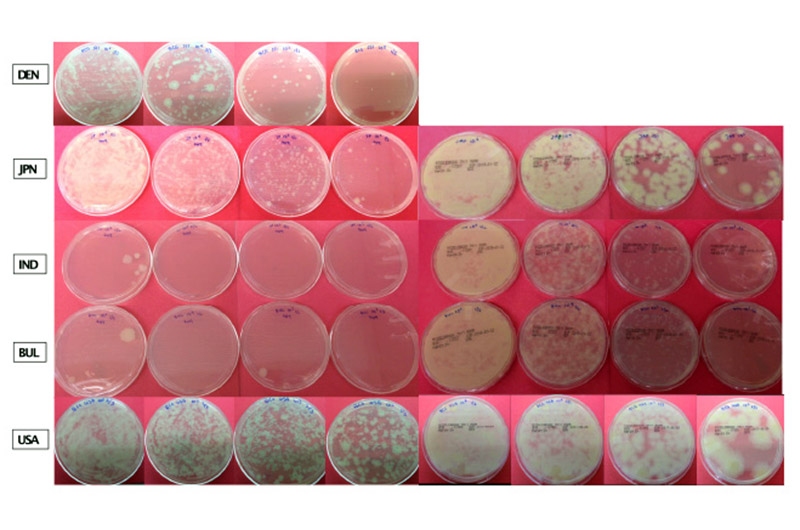
The new study looked at several formulations of the most commonly used licensed BCG vaccines: BCG-Denmark, BCG-India, BCG-Bulgaria, BCG-Japan, and BCG-USA (sourced from the Boston Children’s Hospital pharmacy). The researchers compared several different vials from different manufactured lots of each formulation. They measured how each grew in culture and how many live bacteria each vaccine contained.
“The data consistently shows that the Indian and Bulgarian formulations, both derived from the same mother BCG strain (BCG Russia), have more than 1,000-fold lower growth and fewer live bacteria compared to the others,” says Angelidou. “This is pertinent clinically because there are numerous studies showing that live mycobacteria trigger the immune system in a different way compared to dead mycobacteria; they activate different downstream pathways.”
Cytokines vary by vaccine
The team measured numerous cytokine proteins released from immune cells to fight infection after vaccination, including IL1 beta and interferon gamma (the latter is especially important for TB protection).
“We found differences in terms of the cytokine responses each vaccine triggered,” says Angelidou.
In particular, BCG-India induced significantly less interferon gamma compared to the rest of the strains. The team also found that concentrations of the IL1 beta cytokine, which is heavily involved in boosting overall immunity after BCG vaccination, correlate with the amount of live bacteria contained in the BCG vaccine.
“Upon recognition of a pathogen or vaccine, newborn immune cells are often less able to produce certain cytokines, such as interferon gamma, that are important for an immune response against TB,” says Simon van Haren, PhD, co-senior investigator on this study. “Comparing the ability of each BCG formulation to induce interferon gamma production in newborn immune cells was therefore very important.”
All vaccines not equal
Our findings raise really fundamental questions about whether these BCG vaccines should be considered interchangeable and whether the quality is equivalent because of differences in viability and in the numbers and kind of cytokines induced.
Ofer Levy, MD, PhD
The differences between the strains produced in different parts of the world are mainly due to manufacturing practices.
“The mycobacteria in general are very sensitive to environmental conditions,” says Angelidou, “so any environmental changes in their manufacturing process can really affect the growth of the mycobacteria.”
In their study, the researchers grew the mycobacterium cultures from each vaccine under the same environmental conditions.
“This is key, as it is the first study that directly compares clinically relevant BCG vaccine strains used today in the same lab under the same conditions to see how many bacteria will grow and how they induce immune responses from human newborn white blood cells,” adds Levy.
Large comparative clinical trial needed
This study shows significant differences between the BCG formulations when tested under very controlled conditions. But does it correlate with differences one might see clinically in real world situations?
“We have not proven conclusively which BCG formulation is most effective,” says Levy. “Rather, we present compelling evidence that head to head clinical trials of these very different BCG formulations are urgently needed to define which is most effective.”
###
Simon van Haren from Boston Children’s Hospital is co-senior investigator on this study. Other Boston Children’s Hospital co-authors include Maria-Giulia Conti, Joann Diray-Arce, Mark Liu, Lakshmi Prasad Potluri, Guzman Sanchez-Schmitz, Robert Husson, and Al Ozonoff. Christine Benn from the University of Southern Denmark, Frank Shann from the University of Melbourne, Mihai Netea from the Radboud University, The Netherlands, and Beate Kampmann from the London School of Hygeine and Tropical Medicine are also co-authors.
This work was supported in part by grants from the National Institute of Allergy and Infectious Diseases (NIAID), including those on Molecular Mechanisms of Combination Adjuvants, and the Human Immunology Project Consortium. Support was also provided by the Danish National Research Foundation, the Netherlands Organization for Scientific Research, and a grant from the UK Research and Innovation.
Read more about our work with opioid vaccines, newborn vaccines, and tuberculosis research.
Related Posts :
-

Nanobodies from alpacas could steer immune attacks on influenza
While conventional flu vaccines are designed to anticipate the influenza strains projected to dominate in the next flu season, they’...
-

Zika study reveals how infection can cause microcephaly
Prenatal exposure to viruses capable of infecting the fetal brain, particularly in the first trimester, can cause a range of ...
-

Taming vaccine data: Joann Arce, PhD
Part of an ongoing series profiling researchers at Boston Children’s Hospital. Joann Arce, PhD, is a data tamer — corralling ...
-

Advancing mother-child health globally: Grace Chan MD, MPH, PhD
First in an ongoing series profiling researchers at Boston Children's Hospital. Globally, five million children die annually before the age ...





