Eavesdropping on mitochondria, tissue by tissue
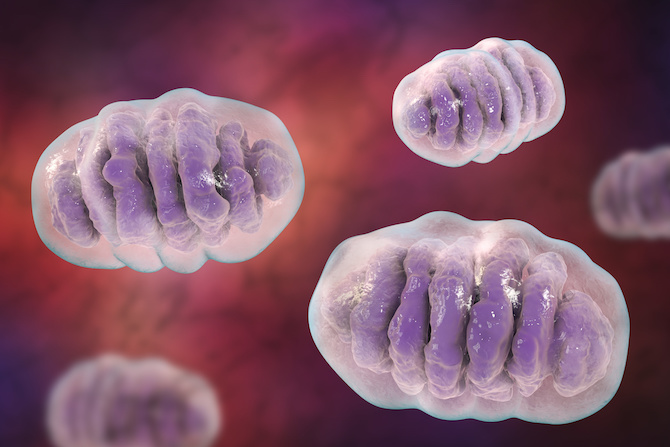
Mitochondria are essential to life: they produce energy, synthesize building blocks critical to cell function and help regulate cellular activity, including programmed cell death. Mitochondrial diseases can cause severe metabolic disorders in children and dysfunctional mitochondria are thought to play a role in cancer, diabetes, heart attack, stroke, Parkinson’s disease and more.
A new research tool offers an unprecedented glimpse at the workings of these tiny, dynamic organelles, and could aid in the study of mitochondrial dysfunction.
Isolating mitochondria in action
To study mitochondria, you often need to isolate them. This is easier said than done. First, mitochondria make up just a tiny part of the cell, making it hard to tell which changes are of mitochondrial origin. Second, you need to isolate them fast enough to accurately capture changes in metabolites and proteins that occur on the fly.
“People have tried shortened isolation methods, like through centrifugation of cells, but you end up with an impure mixture,” says Walter W. Chen, MD, PhD. “We thus wanted to develop a method that is fast but still achieves good purity.”
We can isolate mitochondria in less than 10 minutes and see how they change in response to different conditions.
Chen, a first-year pediatric resident at Boston Children’s Hospital, began working on the problem during his PhD in the lab of David M. Sabatini, MD, PhD, at MIT and the Whitehead Institute for Biomedical Research. He and his colleagues first developed a method for rapid and specific isolation of mitochondria, described in Cell in 2016. The method uses a “MITO-Tag,” a custom protein that localizes to the surface of the organelle, and special antibodies that bind to it.
The antibodies are attached to magnetic beads. “When you break open the cell, you can rapidly capture mitochondria decorated with the MITO-Tag using a magnet,” explains Chen. “With this technique, we can effectively isolate mitochondria in less than 10 minutes, whereas it used to take more than an hour. This has allowed us to look at mitochondrial metabolites and see how they change in response to different conditions. You have more of a window for capturing their biology.”
Location, location, location
Chen and his colleagues performed their original work in cultured cell models, but to better study biology and disease, they wanted to use the MITO-Tag in mice to analyze mitochondria from specific cell types. Previously, this would have involved time-consuming cell sorting techniques that can inadvertently skew the results. So, as recently described in PNAS, they created genetically modified “MITO-Tag Mice,” using a genetic manipulation strategy called Cre/lox.
The mice produce the MITO-Tag themselves, but only in the cell type of interest. This enabled the team to rapidly isolate mitochondria with cell-type-specificity.
“With the MITO-Tag Mice, you can sample tissue, grind it up and use the antibody-conjugated beads to quickly isolate mitochondria from the cells you are interested in, without having to sort the cells ahead of time,” says Chen.
For example, Chen and his colleagues generated MITO-Tag Mice with mitochondria labeled just in liver hepatocytes. They could then take a liver sample and quickly isolate the organelles from the hepatocytes alone. Using this approach, they profiled an array of mitochondrial metabolites under different conditions, such as fasting and feeding, and found metabolic changes that were not evident when the tissue was examined without mitochondrial purification.
A deep dive on organ function
Chen views the mice as a powerful tool for studying complex tissues and organs. Mitochondrial biology is known to vary from cell type to cell type within a tissue. Kidneys, for example, have a variety of cell types exhibiting different amounts of activity in certain mitochondrial metabolic pathways.
“Results from older studies suggest that mitochondria behave differently in different parts of the kidney, so a systematic profiling of mitochondria from specific kidney cell types using the MITO-Tag Mice could be quite interesting,” Chen says.
For the brain, MITO-Tag mice could help elucidate mitochondrial biology in neurons, glial cells and other neighboring cell types.
“Right now, a big focus in biological research is exploring tissue heterogeneity,” says Chen. “This mouse is very well equipped to do that for mitochondria.”
Erol C. Bayraktar (The Rockefeller University) was first author on the PNAS paper. Chen, Sabatini and Kivanc Birsoy (The Rockefeller University) were co-corresponding authors. The study was supported by The Rockefeller University, the National Institutes of Health, the Whitehead Institute for Biomedical Research, the Howard Hughes Medical Institute, the Irma-Hirschl Trust, the American Association for Cancer Research, the Searle Scholars Program, the Sidney Kimmel Foundation, the Pew-Stewart Scholars for Cancer Research Program, the March of Dimes and the Department of Defense.
Related Posts :
-

BRD7 research points to alternative insulin signaling pathway
Bromodomain-containing protein 7 (BRD7) was initially identified as a tumor suppressor, but further research has shown it has a broader role ...
-

When diagnosis is just the first step: The Brain Gene Registry
Through advances in genetic sequencing, many children with rare, unidentified neurodevelopmental disorders are finally having their mysteries solved. But are ...
-

Engineered cartilage could turn the tide for patients with osteoarthritis
About one in seven adults live with degenerative joint disease, also known as osteoarthritis (OA). In recent years, as anterior ...
-

Surgery beats sclerotherapy for rectal prolapse in children ages 5 and older
Rectal prolapse — the protrusion of the lining of a child’s rectum through the anal sphincter — can occur for many ...