‘Face blindness’ may represent a failed brain network — and could shed light on autism
People with prosopagnosia, or “face blindness,” have trouble recognizing faces — even those of close friends and family members. While some people can compensate by using clothing and other cues, face blindness often creates serious social problems. It often becomes apparent in early childhood, but, rarely, people can acquire face blindness later in life after a brain injury.
New research in the journal Brain, studying 44 people who became face-blind after a stroke, provides clues to what goes awry in the brain. It suggests that no one single area is always perturbed in face blindness. Instead, face blindness involves an entire network, where a malfunction in communication among the various components can bring the system down. This potentially opens the door for improving face recognition by tweaking the function of different parts of the network.
Alexander Cohen, MD, PhD, of Boston Children’s Hospital, the lead investigator, believes the findings could also apply to other people with poor face-processing abilities. Specifically, individuals with autism, like the children he sees at Boston Children’s Autism Spectrum Center.
“Over half of children with autism score very poorly on standard tests of face processing,” he says.

Face blindness: From autism to stroke and back again
Cohen began by speculating that autism could be approached not just as a holistic entity, but by studying its individual symptoms. Those symptoms have a better chance of being localized to specific parts of the brain and could perhaps be turned into biomarkers and treatment targets.
That’s where face blindness comes in. It’s easy to test for and common in autism, seen in children as young as 2.
“In eye gaze studies, autistic kids often don’t look at the faces in a video,” says Cohen. “Or they just look at one part, often the mouth, maybe because it’s giving more information about speech. Many find eye contact uncomfortable.”
Data suggest that the worse people with autism are at face processing, the worse their social communication.
“The question is, are face processing deficits causal in autism, or do they result from autism?” says Cohen. “That’s where looking at face processing in people with stroke really helps. They have specific lesions in the brain, and when studying lesions, there is more of a cause and effect relationship. If you find an abnormality involving the same brain areas in a child with autism, there’s a much higher chance that the abnormality may be driving the face processing deficit.”
Mapping brain networks
Face blindness has been linked to the brain’s right fusiform face area (FFA), but not everyone with face blindness shows damage there. To investigate further, Cohen searched the medical literature and identified 44 people from 19 studies who developed face blindness after a stroke and who also had brain MRI data available.
Of the 44 lesions causing face blindness, only 29 actually involved the right FFA. What about the rest? That’s where brain networks come in.
Cohen used a new method, developed by senior study author Michael Fox, MD, PhD, of Beth Israel Deaconess Medical Center, that uses a large-scale map of brain networks, or a “connectome,” based on data from noninvasive functional MRI imaging. The map shows the relationships between different brain areas, and with it, Cohen could determine which networks were consistently injured in stroke patients that developed face-blindness.
In the stroke patients, all 44 lesion locations, including the 15 non-FFA lesions, were in areas that were functionally connected to the right FFA, meaning that these regions are typically used when the FFA is being used, and become quiet when the FFA is quiet.
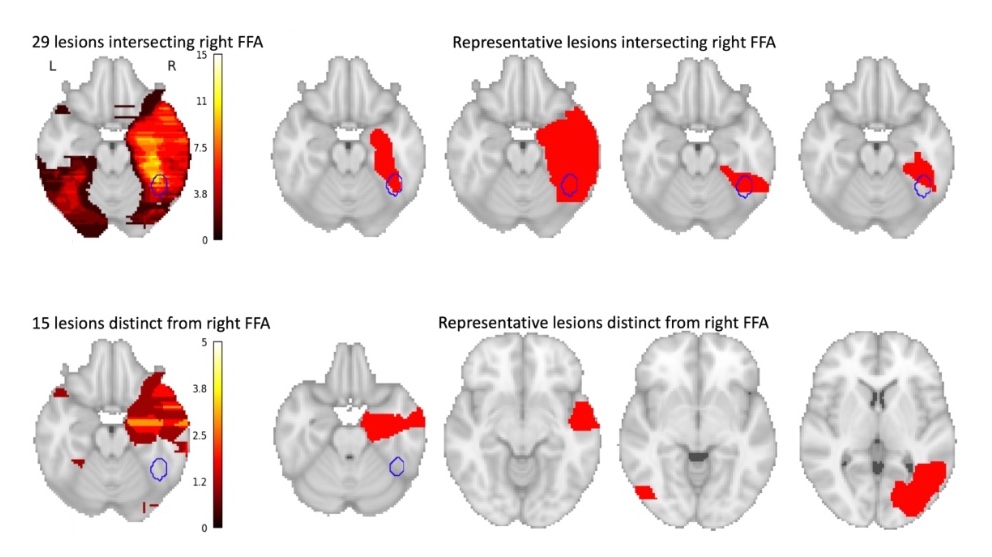
(Cohen AL; et al. Brain 2019, DOI: 10.1093/brain/awz332)
Surprisingly, all of these lesion locations were also negatively connected with four additional areas in the left frontal cortex, shown below. This means that activity in these regions goes down when activity in the lesion locations goes up, and vice versa. Intriguingly, all four of these areas belong to the left frontoparietal control network, which attends to specific features of a visual stimulus.
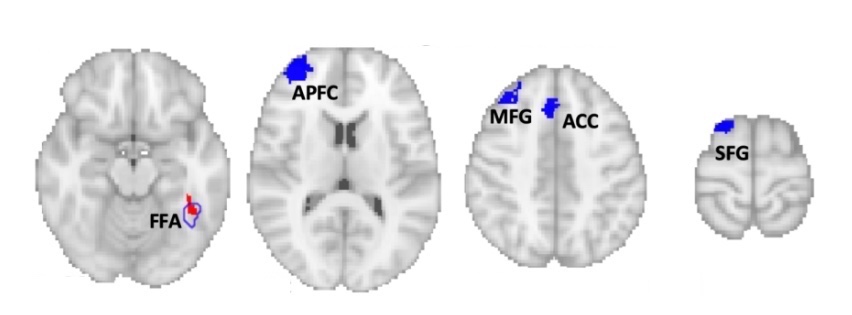
Based on the findings, Cohen speculates that face recognition involves two distinct brain networks. What’s not yet clear is whether face blindness requires disruption of both networks, or whether it results from an imbalance between the two.
“Maybe in face blindness, you’re using too much detail information, and are not looking at face as a whole,” Cohen says. “That exact imbalance is something that has been seen in autism. Or, maybe you need both kinds of information to recognize a face.”
Mapping face processing in tuberous sclerosis
Based on the stroke data, Cohen now plans to delve back into the neuroanatomical basis of face processing problems in autism spectrum disorder. Under a $100,000 two-year research award from the Child Neurology Foundation, Cohen will study children with tuberous sclerosis complex (TSC), a genetic syndrome that often includes autism and atypical face processing.
Most children with TSC have tubers (noncancerous tumors) in the cortex of their brains. These are thought to affect the function of nearby brain tissue and may provide insight into where symptoms come from, similar to studying strokes in adults. To test this, Cohen recently led a study of another common symptom in TSC: infantile spasms. It found that these children’s tubers, while located in different parts of the brain, are all part of a network involving the globus pallidus.
Do tubers affect face recognition as well? Under his new grant, Cohen will recruit up to 80 children with TSC, locate their brain tubers, administer tests of face processing, and map their brain networks. He will then compare the findings with his data from the stroke study to see if common brain areas are involved.
Could face blindness be treated?
Eventually, Cohen hopes to do a larger study of brain connectivity and face processing in children with garden-variety (non-syndromic) autism.
“Just as many disorders can be affected by multiple genes, it may take a whole network of brain regions to cause a symptom,” he says.
If all roads in that network lead to a specific area in the brain, that area could potentially be treated by methods to increase or decrease its activity. Even addressing face processing in isolation could improve quality of life in children with autism — and others with face blindness.
“We could try to modulate activity in that spot — with novel therapies like transcranial magnetic stimulation or functional-MRI-based neurofeedback — and see if it affects behavior,” Cohen says. “If this works, we aim to expand this methodology to many other symptoms in autism as well.”
The stroke study was funded by the National Institutes of Health (T32MH112510, F32EY023479, K23NS083741), a Canada Research Chair (950-228984), the Canadian Institutes of Health Research (MOP-102567), the Marianne Koerner Chair in Brain Disease, the Sidney R. Baer, Jr. Foundation, the Dystonia Foundation, and the Nancy Lurie Marks Foundation.
To inquire about the tuberous sclerosis (TSC) study, contact Alexander.Cohen2@childrens.harvard.edu.
Related Posts :
-

Medical care for youth with neurodevelopmental disabilities: A call for change
According to national data, one in six children has a neurodevelopmental disability (NDD) such as autism, intellectual disability, or ADHD. ...
-

Exploring brain operations: Making decisions, snapping to attention, and forming memories
How do our brains snap to attention and orient us to the outside world — like when we’re sound asleep ...
-

The journey to a treatment for hereditary spastic paraplegia
In 2016, Darius Ebrahimi-Fakhari, MD, PhD, a neurology fellow at Boston Children’s Hospital, met two little girls with spasticity and ...
-

It takes a village and the world: Tariq’s care for Tourette syndrome
When your child is sick but you can’t figure out the cause or how to fix it, it can ...





