As COVID-19 fuels opioid deaths, researchers look to create an anti-opioid vaccine
A project that began one year ago at Boston Children’s Hospital to develop an anti-opioid vaccine is starting to bear its first fruits. A team of addiction experts and vaccine developers across the Boston Children’s research community were recently awarded a $25 million research contract from the NIH’s Helping to End Addiction Long-term Initiative, or NIH HEAL Initiative, to develop a vaccine to prevent overdose in opioid users and conduct a clinical trial when it is ready.
The effort is led by the principal investigator team of Ofer Levy MD, PhD and David Dowling, PhD, of Boston Children’s Precision Vaccines Program (PVP).
Opioid deaths rising during COVID-19
The need for an anti-opioid vaccine has only grown in the COVID-19 pandemic.
Key takeaways
Opioid-related deaths are on the rise during the COVID-19 pandemic.
The Precision Vaccines Program is developing a new vaccine against overdose from fentanyl-containing drugs.
The team has been awarded a $25 million grant from the NIH to create the vaccine and conduct a human clinical trial.
“We know that substance use rates are rising,” says Sharon Levy, MD, MPH, director of Boston Children’s Adolescent Substance Use and Addiction Program (ASAP).

Every day about 130 Americans die of opioid overdose and the numbers have been increasing since the onset of COVID-19 pandemic. According to the American Medical Association, more than 40 states have reported increases in deaths from opioids. A June survey of US adults released by the Centers for Disease Control and Prevention, found that 13 percent of respondents reported starting or increasing use to deal with stress or emotions related to COVID-19.
Preventing fentanyl overdose deaths

Fentanyl is a synthetic, highly potent opioid that is often added to other drugs like heroin, cocaine, and sometimes even marijuana. The drug is so powerful that even a small amount can suppress one’s ability to breathe. The proposed vaccine is intended for individuals who have opioid use disorder (OUD) and might be exposed to fentanyl — either directly or through contamination — or are trying to get off opioids.
The goal is to give opioid users a fentanyl-based vaccine that will stimulate their bodies to make antibodies against it. The antibodies would then bind to fentanyl in the blood, thereby blocking it from reaching the brain, where it suppresses respiration.
Adding a needed boost for immunity
“Opioids are small molecules that only induce a significant immune response when attached to a larger carrier such as a protein,” says Dowling, who heads the PVP’s Adjuvant and Discovery and Development Laboratory.
To overcome this challenge, Dowling has been leading the effort to identify a protein carrier plus adjuvant combination to add to an anti-opioid vaccine.
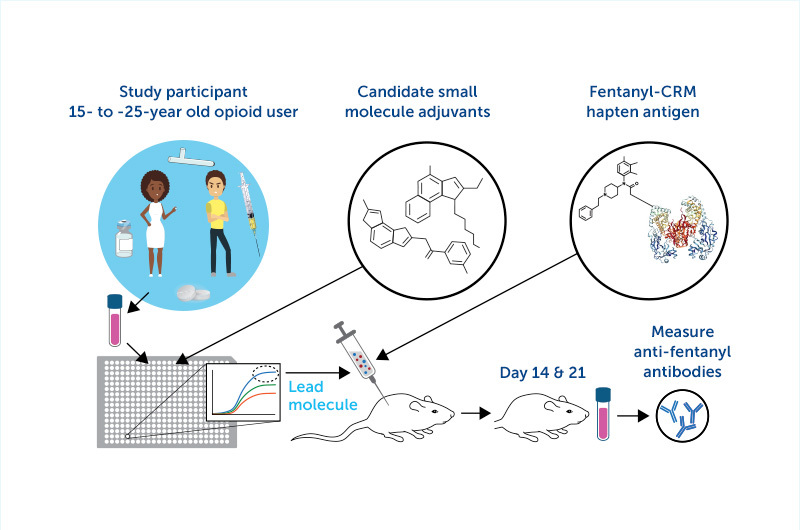
“Adjuvants are the molecules we add to a vaccine that act like rocket fuel to induce a stronger immune response,” says Dowling. “We are looking for adjuvants that work best in those with a history of opioid use, as their immune system may be unique due to the effect of opioids.”
Looking to the first human clinical trial
As we mentioned in our first story about this effort, Sharon Levy’s ASAP team has been enrolling youth who have OUD in the project. Using blood samples from volunteers, researchers have been testing which adjuvants best activate white blood cells in those people and compared them with cells from people without OUD.
“Overall, we believe a precision vaccinology approach that tailors an adjuvant system to those with OUD is an innovative way to accelerate vaccine development for this vulnerable population,” says Ofer Levy.
While the team has already identified several candidate adjuvants, they expect to find others with funding from this new award. Once the best combination of adjuvant and vaccine is identified — a goal that will take time as it moves through preclinical testing prior to studies in animals — it will be tested in humans as part of a clinical trial based at Boston Children’s.
Will users take it, though?

As the work proceeds, Elissa Weitzman, ScD, MSc, of the Division of Adolescent and Young Adult Medicine and principal investigator of the Weitzman Lab, is simultaneously investigating the attitudes and beliefs of at-risk youth and other stakeholders toward an anti-fentanyl vaccine.
“Early understanding of the social-behavioral factors that might impact vaccine uptake is vital to success since we can consider them in the development work,” says Weitzman.
Other members of the Boston Children’s research community contributing to the project include Simon van Haren, PhD; Hanno Steen, PhD, of the Department of Pathology; and Cindy Williams, DNP, RN, and Andrew Place, MD, MPH, both of the Institutional Centers for Clinical and Translational Research (ICCTR).
External collaborators include Jay Evans, PhD of Inimmune (Missoula, MT), Tom Kosten, MD, Therese A. Kosten, PhD, Colin Haile, M.D., PhD and Greg Cuny, PhD of the University of Houston (Houston, TX), and Christopher Chen, PhD of Texas Biomedical Research Institute.
###
This project has been funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. 75N93020C00038 as described in Article H.36 of the contract.
The NIH HEAL Initiative is an aggressive, trans-NIH effort to speed scientific solutions to stem the national opioid public health crisis. Launched in April 2018, the initiative is focused on improving prevention and treatment strategies for opioid misuse and addiction and enhancing pain management. For more information, visit: https://heal.nih.gov.
Read more about vaccine research from the Precision Vaccines Program.
Related Posts :
-

A vaccine to prevent opioid overdose?
Sharon Levy, MD, MPH, who directs the Adolescent Substance Use and Addiction Program (ASAP) at Boston Children’s Hospital, was ...
-

Opioids for acute pain in kids: Four things to know
According to the Centers for Disease Control and Prevention, an estimated 130 Americans die every day from an opioid overdose. Opioid ...
-

High numbers of youth report using prescription opioids in the past year
A new analysis of data from the National Survey on Drug Use and Health finds a surprisingly high prevalence of ...
-

Concerning rates of opioid prescribing to teens and youth
Teens and young adults are notably susceptible to misusing opioids and becoming addicted once exposed. Opioids should be used with ...