Stem cell workaround cracks open new leads in Diamond Blackfan anemia
Diamond Blackfan anemia (DBA) has long been a disease waiting for a cure. First described in 1938 by Louis K. Diamond, MD, of Boston Children’s Hospital and his mentor, Kenneth Blackfan, MD, the rare, severe blood disorder prevents the bone marrow from making enough red blood cells. It’s been linked to mutations affecting a variety of proteins in ribosomes, the cellular organelles that themselves build proteins. The first mutation was reported in 1999.
But scientists have been unable to connect the dots and turn that knowledge into new treatments for DBA. Steroids are still the mainstay of care, and they help only about half of patients. Some people eventually stop responding, and many are forced onto lifelong blood transfusions.
Researchers have tried for years to isolate and study patients’ blood stem cells, hoping to recapture the disease process and gather new therapeutic leads. Some blood stem cells have been isolated, but they’re very rare and can’t be replicated in enough numbers to be useful for research.
Induced pluripotent stem (iPS) cells, first created in 2006 from donor skin cells, seemed to raise new hope. They can theoretically generate virtually any specialized cell, allowing scientists model a patient’s disease in a dish and test potential drugs.
There’s been just one hitch. “People quickly ran into problems with blood,” says hematology researcher Sergei Doulatov, PhD. “iPS cells have been hard to instruct when it comes to making blood cells.”
The next best thing
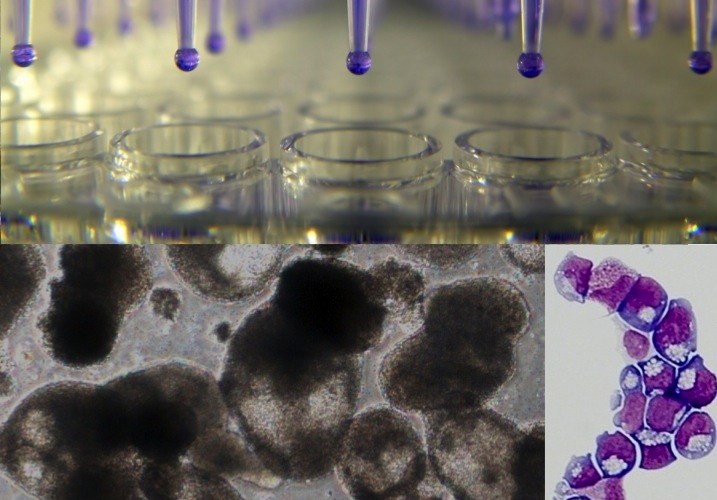
But Doulatov and his colleagues in the labs of George Q. Daley, MD, PhD and of Leonard Zon, MD, both part of Boston Children’s Stem Cell Research program, came up with the next best thing to blood stem cells. By adding five reprogramming factors, they got iPS cells to make so-called hematopoietic progenitor cells, shown at right.
Hematopoietic progenitors share many properties with blood stem cells and are readily multiplied in a dish. Most importantly, they neatly model DBA: Like the patients from whom they’re derived, they cannot generate the erythroid cells that make red blood cells.
In parallel, the Zon Lab had developed zebrafish models of DBA. Together, the two labs were well equipped to try to solve the red-cell problem.
DBA drug discovery
As reported yesterday in Science Translational Medicine, the Daley-Zon team loaded the hematopoietic progenitors into a high-throughput drug screening system complete with a library of 1,440 chemicals. Since drug screens are typically done in duplicate, this required tens of thousands of wells. But having enough cells was no longer a problem.
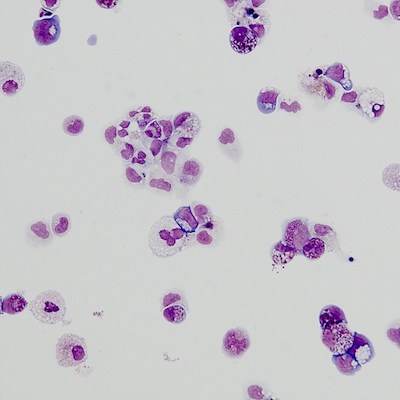
The chemical screen produced several “hits”: in wells loaded with these chemicals, erythroid cells began appearing. One compound, SMER28, had an especially potent effect, so was put through additional testing.
When live zebrafish and mice with DBA were treated with SMER28, they began making erythroid progenitor cells that in turn churned out red blood cells, reversing or stabilizing anemia. The same was true in cells from patients with DBA, transplanted into mice.
The researchers believe SMER28 or a similar compound might offer another much-needed option for DBA. “This is the first time iPS cells have been used to identify a drug to treat a blood disorder,” says Doulatov, who now heads a lab at the University of Washington.
The quest continues
Zon and Daley have been awarded NIH funding from the National Heart, Lung and Blood Institute’s Progenitor Cell Translational Consortium to further explore several promising compounds identified through the drug screen.
In the meantime, Doulatov plans to further explore SMER28’s biological role. SMER28 turns out to activate a so-called autophagy pathway that recycles damaged cellular components; in DBA, it appears to act on erythroid progenitors. Doulatov plans to further explore how this interferes with red blood cell production.
Linda Vo in the Daley Lab and Elizabeth Macari, PhD in the Zon lab were co-first authors on the paper with Doulatov.
Related Posts :
-

Microvillus inclusion disease: From organoids to new treatments
Microvillus inclusion disease (MVID) is a rare type of congenital enteropathy in infants that causes devastating diarrhea and an inability ...
-

Blood donations help Kit manage Diamond-Blackfan anemia — so she can dance, sing, and enjoy life
Every month, Kit Murdoch needs a blood transfusion to stay alive. The 2-year-old has Diamond-Blackfan anemia, a rare ...
-

Sickle cell gene therapy and boosting fetal hemoglobin: A 75-year history
Ed. Note: This post updates an earlier post from 2018. In a landmark decision today, the Food and Drug Administration (FDA) ...
-

The clot thickens: Kellie Machlus, PhD
Part of an ongoing series profiling researchers at Boston Children’s Hospital. Platelets are the bandages of our blood, forming ...





