What’s the difference between the COVID-19 vaccines?
After a long, difficult year, the world had reason to celebrate in late 2020 when the U.S. Food and Drug Administration (FDA) authorized two COVID-19 vaccines for emergency use. Many more COVID-19 vaccines are in various stages of development. Several of these are in the final stage of clinical trials and could be submitted for formal authorization as early as this spring.
Why are there so many vaccines, and how are they different? Two specialists in infectious disease at Boston Children’s Hospital answer these questions, describe the safety measures each vaccine undergoes, and what needs to happen before we can truly declare victory over COVID-19.
Why are there so many COVID-19 vaccines?

“COVID-19 is a global health emergency,” explains Dr. Ofer Levy, director of the Precision Vaccines Program. Throughout this past year, laboratories around the world went to work to develop COVID-19 vaccines and test their safety and effectiveness. “We didn’t know which vaccines would work at the start of this pandemic. If we tested them one at a time, it could have taken decades to find a vaccine that worked.”
The first vaccine to receive authorization in the U.S., developed by the drug company Pfizer, was 95 percent effective in preventing mild to moderate COVID-19 disease in clinical trials. The second vaccine, developed by Moderna, was 94.5 percent effective. While no one could have predicted the first vaccines would work so well, having several in production at once means more people can be vaccinated more quickly.
Are the COVID-19 vaccines safe?
“Despite the accelerated path to authorization, each vaccine has to undergo rigorous testing to determine their safety,” says Dr. Richard Malley, senior physician in the Division of Infectious Diseases whose research focuses on vaccine development. “In addition to the companies that developed them and scientists at the FDA, each vaccine is evaluated by an independent committee. The individuals on these committees are not beholden to anyone.”
What about early reports of allergic reactions?
“In a few rare cases, people have experienced severe allergic reactions to the vaccines,” says Dr. Levy, who serves on the FDA vaccine advisory committee that reviewed the Pfizer and Moderna vaccines. “We are continuing to collect data so we can understand these reactions and what causes them,” he says.
In the meantime, the Centers for Disease Control and Prevention (CDC) recommends that anyone who gets vaccinated remain at the facility for 15 to 30 minutes so health care workers can watch for signs of an allergic reaction and provide prompt treatment if necessary.
How do the different COVID-19 vaccines work?
“In a broad sense, the vaccines that have been studied most extensively to date trigger the immune system to target the spike proteins on the surface of the coronavirus,” says Dr. Malley. Different COVID-19 vaccines accomplish this in different ways.
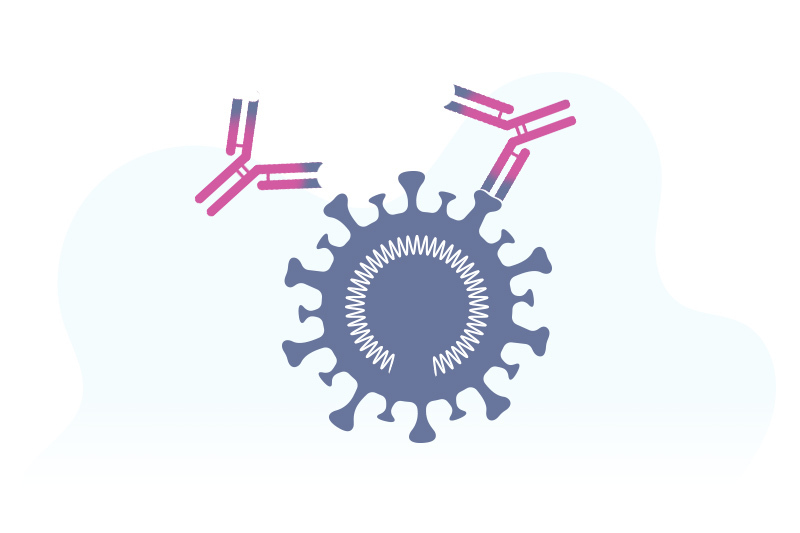
mRNA vaccines use a strip of genetic material called mRNA. Once the mRNA enters a cell, it triggers the cell to build copies of the spike proteins. The immune system learns to recognize these spike proteins through the production of antibodies that block the virus from entering healthy cells. Both the Pfizer and Moderna vaccines work this way.
Viral vector vaccines rely on another virus, called an adenovirus. The adenovirus is engineered to encode the spike proteins and create immunity. Several viral vector vaccines are in the final stage of clinical trials and could be could be submitted for authorization for use in the United States in the first half of 2021.
Purified protein vaccines take a more traditional approach. These vaccines use proteins to stimulate an immune response.
Whole, inactivated virus vaccines use dead versions of the virus to generate immunity.
How are the vaccines different?
One important difference has to do with how the vaccines are stored. The Pfizer vaccine requires special freezers that can maintain a temperature of negative 94o F. The Moderna vaccine needs to be frozen at negative 20o F, roughly the same temperature as a standard home freezer. Most of the other vaccines currently in clinical trials are stable at 40o F, standard refrigeration.
Some large institutions, like Boston Children’s, have deep freezers cold enough to store the Pfizer vaccine. Other vaccines will be more practical in many smaller practices and rural communities.
Does getting a COVID-19 vaccine mean I can stop wearing a mask?

Not yet. “Whether you’re vaccinated or not, your life won’t change right now,” says Dr. Malley. To protect the people around you, it will be important to continue precautions such as mask-wearing and avoiding indoor gatherings for a while longer.
“The Pfizer and Moderna vaccines both proved highly effective in protecting people from developing COVID-19,” he explains. “While there are some promising signs, we don’t know yet if the vaccines make people less likely to transmit the virus to others.” That’s something both Pfizer and Moderna will study over the next several months.
It will also take time to reach a point of herd immunity, a point at which enough individuals in the population have been vaccinated to stop the virus from spreading. As with many other infectious diseases, vaccine-induced herd immunity will be a big step toward returning to life as we knew it before the pandemic.
Do the vaccines have side effects?
Some people have side effects such as soreness at the injection site, headache, fatigue, and low-grade fever. Typically, such side effects last only a day or two.
Anyone who has been vaccinated can help the FDA and CDC monitor side effects through an app called v-safe. If a person experiences side effects, they can easily report them using their phone. “This is one of many surveillance tools that will allow us to track the safety of coronavirus vaccines over time.” says Dr. Levy.
Learn more about Boston Children’s Hospital’s response to COVID-19.
Related Posts :
-

The new COVID-19 vaccines: Will they be safe?
Just within the past week, pharmaceutical companies have announced encouraging news about two COVID-19 vaccines in the final development phase. ...
-

Testing the COVID-19 vaccines in kids: Is it safe?
The drug company Pfizer recently announced they had opened their clinical trial of the COVID-19 vaccine to teens as young ...
-

Getting to a COVID-19 vaccine as fast and as safely as possible
The novel coronavirus is not expected to disappear anytime soon. With physical distancing, virus testing, contact tracing, and potentially new ...
-

Designing a coronavirus vaccine for next year – and the years beyond
As the number of coronavirus infections swell daily across the globe, strategies for developing a safe and effective vaccine are ...