Diving into the dark side of ependymoma

Mariella Filbin, MD, PhD, a neuro-oncologist at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, is driven by a desire to find new therapies for some of the hardest-to-treat pediatric brain tumors. At the core of her work is an effort to uncover the events that shape tumor development.
· Aggressive ependymoma tumors are stuck in an undifferentiated, stem-cell-like state.
· Single-cell RNA sequencing reveals a genetic program associated with aggressive, undifferentiated tumors.
· Ependymomas are driven by epigenetics, rather than DNA changes.
During her medical training, Filbin recognized early on the importance of defining and targeting the transcriptional networks, or genetic regulators, that underlie tumor growth.
In 2014, she directed her focus to ependymoma. She was inspired by a patient who, at 3 years old, was diagnosed with a particularly aggressive form of the brain tumor. This form has a very poor prognosis, even with extensive treatment interventions.
There was no way to tell at the time what subtype of ependymoma this child had. All Filbin knew was there was a 50/50 chance the child would relapse.
“It’s a conundrum,” she says. “One subtype of ependymoma is treatable — we remove it, radiate it, and there is no other treatment needed — while the other one will ultimately recur despite intensive therapy.”

What makes these tumor cells more aggressive and others treatable?
In a new paper published today in Cancer Cell, Filbin’s team set out to answer this very question. Her lab studied 28 tumor samples, some initial and some recurrent, in 24 patients.
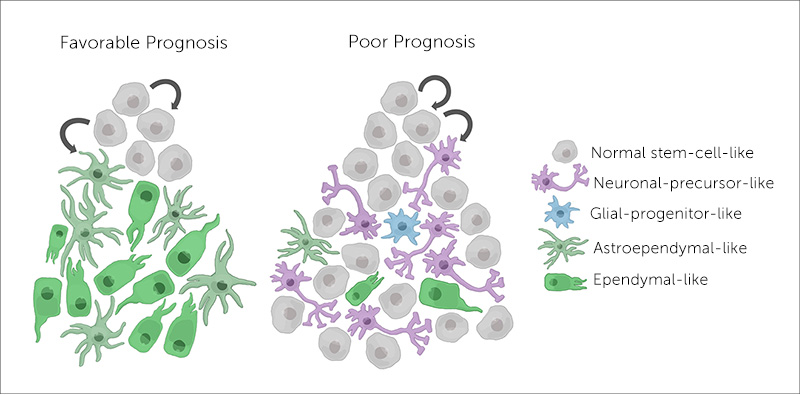
Ependymoma cells arise from stem cells, unspecialized cells that can form a range of different cell types in the brain. Some get the message to differentiate and become almost normal neuronal and glial cells. But others remain undifferentiated — stuck in the stem cell stage. Filbin’s team found that tumors with these undifferentiated cells are 50 percent more likely to relapse. That was the case with Filbin’s young patient.
Based on her team’s findings, Filbin describes ependymoma as having a hierarchy of cell types. “They span undifferentiated and differentiated tumor cell states across all major molecular groups,” she says. The undifferentiated cells continue to grow and spread, making a tumor more difficult to treat and more likely to recur.
Her next question: What inciting event causes cells’ paths to diverge?
Surprisingly, no recurring DNA mutations have yet been linked with the more lethal tumor cell pathway. With this in mind, Filbin hoped to identify events occurring during steps in cell development and maturation that were associated with stem cells’ failure to mature.
“A cancer that’s driven by a lot of DNA damage, like melanoma or lung cancer, is on one end of the spectrum,” says Filbin. “Ependymoma is on the other end. It’s the one tumor we think is epigenetically driven, meaning it does not change the DNA sequence, but instead changes how cells interpret genes.”
Single-cell sequencing reveals RNA fingerprint
By applying single-cell RNA sequencing, Filbin and colleagues were able to identify a specific genetic program associated with undifferentiated stem cells. They suggest that this unique transcriptional signature could help predict which tumor a patient has and the outcome.
The team further established that the cell of origin differs depending on where the ependymoma arises in the brain and central nervous system. Filbin notes that the more aggressive tumors, similar to the “good prognosis” tumors, develop in the cerebral hemispheres and posterior fossa, at the bottom of the skull.
More research from the Filbin lab.
“We think that the underlying stem cell type determines partly what happens down the line, and that the tumor is then further shaped by microenvironmental factors,” Filbin says.
While Filbin’s findings offer unprecedented and invaluable insight into the tumor’s etiology, she acknowledges that there is more work to be done. The relapse of her young patient is just another reminder of the need.
“For the first time we’re understanding what cells really do in a tumor that’s enigmatic and very different from all other cancers,” she says. “We’re still scratching the surface, but we need new treatment strategies as soon as possible.”
Learn more about the Brain Tumor Center at Dana-Farber/Boston Children’s.
Related Posts :
-

Making genome sequencing a first-line test in rare disease
Children with rare diseases often undergo years of medical visits and genetic testing before they get a diagnosis. Over the ...
-

Boosting vaccines for the elderly with ‘hyperactivators’
As we age our immune systems start to flag, leaving us more susceptible to cancer and infections — and less responsive ...
-

Two rising stars in kidney genetics: Nina Mann and Amar Majmundar
A healthy, functional kidney must maintain a delicate balance of water, nutrients, and electrolytes so it can properly filter the ...
-

Whether she’s embracing school, sports, or music, Lindsey shows how Williams syndrome can be managed
One of the first things Lindsey Franco will tell you is, “I like being me. I like being happy.” The 19...





