Missed signals? A new way we vary from each other biologically
Genetics has made huge strides over the past 20 years, from the sequencing of the human genome to a growing understanding of factors that turn genes on and off, namely transcription factors and the DNA “enhancer” sequences they bind to. New research from Boston Children’s Hospital introduces another previously unknown layer of human genetics. It finds genetic variation in a gene’s ability to react to chemical signals from the outside.
Key takeaway
This study reveals a new, previously unknown layer of human genetics: genetic variation in how readily our genes respond to outside signals that turn them on or off. This may help explain individual differences in physiology, disease susceptibility, and drug responses.
The study, published today in Nature Genetics, focused on genetic variations in red blood cell traits. But Leonard Zon, MD, director of the Stem Cell Research Program at Boston Children’s, believes the findings apply to any tissue or organ in the body. They may help explain why some people are more or less susceptible to disease, why organs function differently, and why some people respond more to drugs than others.
“We found that human genetics determine, on a gene to gene basis, whether a cell will respond to an outside signal,” says Zon, who is also affiliated with Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, the Howard Hughes Medical Institute, and the Harvard Stem Cell Institute. “We believe that many genetic conditions are due to a defect in this response — a ‘signalopathy.’”
When DNA enhancers can’t receive signals
Zon and his team used data from recently published genome-wide association studies (GWAS). These studies scan the genomes of large numbers of people to find genetic variants associated with a trait or disease. In this case, the team sought out genetic variants associated with seven red blood cell traits, such as size and hemoglobin concentration.
People vary in how much signaling can happen in an individual gene.”
– Leonard Zon
As it turns out, many variants tied to these traits mapped to a small subset of gene enhancers. These enhancers bind to two types of gene regulators: master transcription factors that regulate which type of blood cell is being made, and signaling transcription factors that coordinate responses to signals from outside the cell.
When Zon and colleagues looked at blood cell progenitors in the lab, they found that many of the variants altered the DNA sequences of enhancers to which signaling transcription factors bind. This, they showed, prevented the factors from binding to the enhancer. That missed signal prevented adjacent genes from turning on — genes that normally would turn on in response to cues driving red blood cell maturation.
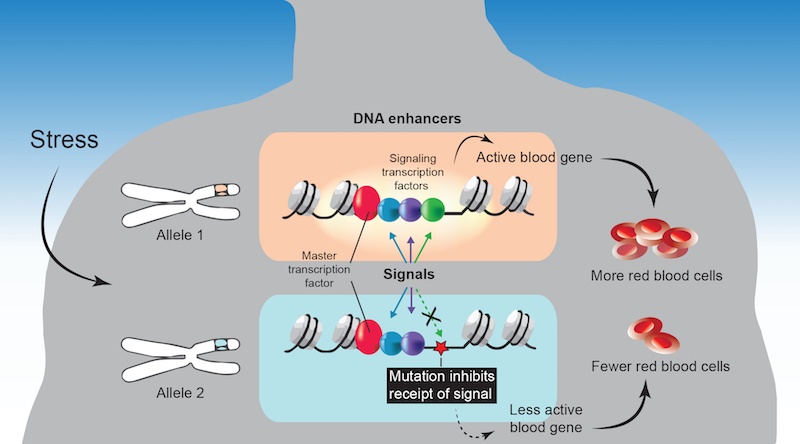
“A mutation in this code kicks the signaling factor off the DNA, because it doesn’t have a binding site,” elaborates Avik Choudhuri, PhD, first author on the study, who is also in the Harvard Department of Stem Cell and Regenerative Biology (HSCRB). “This renders an important blood gene periodically unresponsive to the signal. Such an abnormal response, especially under stress, would cause tissue damage and disease over time.”
A better understanding of human traits
GWAS studies have mapped many traits to variations in DNA enhancer sequences. But no one had ever shown that traits can be altered because of a failure of signals to get through.
“This wasn’t known before,” says Zon. “If we want to better understand human variation, we have to find those regions in the genome, in every tissue, that are receiving transcriptional signals from outside the cell. People vary in how much signaling can happen in an individual gene.”
If a mutation shuts down one signal binding site, you might be able to rescue the disease by sending a different signal.”
– Leonard Zon
The study relied heavily on computational analysis of genome-wide data. “Observing this pattern — trait-associated variants especially impacting DNA landing sites for signaling transcription factors — required that we integrate many datasets and data types from a variety of biological systems,” says co-first author Brian Abraham, PhD, of the Whitehead Institute for Biomedical Research and now at St. Jude Children’s Hospital.
New treatment targets?
Looking to the future, Zon believes the team’s work will open up new therapeutic opportunities. He envisions a computational analysis of a specific gene, its enhancers, and sites on the enhancers where signaling chemicals bind. Such analysis could predict which signals a given cell type will be most likely to “hear,” guiding the choice of drugs. A company Zon co-founded in 2018, CAMP4 Therapeutics, is focused on drug discovery through this kind of mapping.
“You could just look at the DNA code and know what drug to give,” Zon speculates. “If a mutation shuts down one signal binding site, you might be able to rescue the disease by sending a different signal.”
Alternatively, scientists could design therapies that alter the enhancer DNA itself so that it can receive incoming signals, or therapies that turn on the enhancer independent of the signaling pathway, says Zon.
Choudhuri, Abraham, and Eirini Trompouki, PhD, of Boston Children’s were co-first authors on the paper. (Trompouki is now at the Max Planck Institute.)
The study was supported by the National Institutes of Health, the Max Planck Society, The Fritz Thyssen Stiftung, a Marie Curie Career Integration Grant, the Centre for Integrative Biological Signalling Studies, Deutsche Forschungsgemeinschaft, the Hope Funds for Cancer Research, and the American Lebanese Syrian Associated Charities.
Zon is founder and stockholder of Fate, Inc., Scholar Rock, CAMP4, and Amagma Therapeutics. Coauthor Richard Young is founder of Syros Pharmaceuticals and CAMP4. Adams is a shareholder in Syros Pharmaceuticals. The other authors declare no competing interests.
Explore more discoveries from the Stem Cell Research Program.
Related Posts :
-

A case for Kennedy — and for rapid genomic testing in every NICU
Kennedy was born in August 2025 after what her parents, John and Diana, describe as an uneventful pregnancy. Soon after delivery, ...
-

The journey to a treatment for hereditary spastic paraplegia
In 2016, Darius Ebrahimi-Fakhari, MD, PhD, then a neurology fellow at Boston Children’s Hospital, met two little girls with spasticity ...
-

New research paves the way to a better understanding of telomeres
Much the way the caps on the ends of a shoelace prevent it from fraying, telomeres — regions of repetitive DNA ...
-

New research sheds light on the genetic roots of amblyopia
For decades, amblyopia has been considered a disorder primarily caused by abnormal visual experiences early in life. But new research ...